Glenn Randall
I-Search Rough
Draft
Week # 13
Fluorocarbon
Ski Waxes
When I started skiing as a small
child, I could never have imagined what skiing would do to me. The first time I skied, I was probably no older
than two and my parents had merely put skis on my feet and let me wander around
the house with them on. I'm not sure
when exactly I got on snow, but I know that ever since then I've been hooked.
Even
as a small child, I had a huge urge to race.
My parents told me that I could race as soon as I could climb the
biggest hill on the local 1 km course.
While I now know that the hill was very small and gradual, at the time
it was a momentous trek, seeming more like cycling's L’Alpe D'Huez than the
small hill that it actually is.
Ever
since I could climb the hill and have been allowed to race, I have loved
it. As I got better and learned about
topics such as force, energy, and friction, I began to wonder why a ski works,
and what sort of magic makes some waxes faster than others. Since I have been a small child, I have grown
up around skis and ski waxes, but I never knew why or how they worked. The purpose of this paper is to find out what
makes skis fast, how fluorocarbon waxes differ from other types of wax, and
what makes fluorocarbons fast.
There
are two types of ski waxes, glide and kick.
Kick wax is used in classic skiing, but not in skate skiing. It is applied to the ski to keep the ski from
sliding backwards, giving the ski what skiers call "kick." This term merely describes how well the ski
holds to the snow when the skier kicks backwards on the snow for foreword
propulsion. It is relatively easy to
imagine how a stickier kick wax works better for icy snow, and a less sticky
kick wax works better for slower snow.
Glide
wax is what makes the ski fast. It is
more complicated. Most glide waxes have
to be melted into the base of the ski.
Ski bases have been made of polyethylene for quite some time (Talbot
8). Polyethylene skis are translucent (Onion). Eventually, people figured out that graphite
could be added to ski bases (Charonnat, Understanding Glide Waxes). Graphite is a soft black form of carbon
(Advanced Dictionary). It reduces static
electricity and transfers heat to the center of the ski, speeding it up
(Charonnat, Understanding Glide Waxes).
Modern ski bases have a microscopic structure made up of many hair-like
structures. This creates places where
ski wax can be absorbed (Talbot 8).
Most
people think of snow and ice as being characteristically slick. It is not.
In fact, at cold enough temperatures, people can walk on ice without
fear of slipping. This is because under
the pressure of a foot or a ski a thin layer snow and ice tends to melt. A 100 lb skier going 60 mph down a hill
produces energy equivalent to if they had three 100 W light bulbs on the bottom
of their ski (McKibben). This thin layer
lubricates the area between the ice and the foot (Onion). When trying to get to a car on a snowy day,
this may be bad, but when trying to ski fast on snow, this is a very good
thing.
Cheap
glide waxes that can be bought in the store are usually hydrocarbons
(Charonnat, Fluorinated Waxes). A
hydrocarbon is a chain of carbon atoms with hydrogen atoms bonded to the carbon
atoms (Charonnat, Fluorinated Waxes).
Two hydrogen atoms are bonded to every carbon atom throughout most of
the hydrocarbon molecule. Hydrocarbons
exist in many forms. Polyethylene, the
ski base material, is a very long hydrocarbon, consisting of thousands of
carbon atoms. This length gives it a
very high melting point and an even higher boiling point. On the other extreme, propane is a
hydrocarbon consisting of three carbon atoms (Talbot 8).
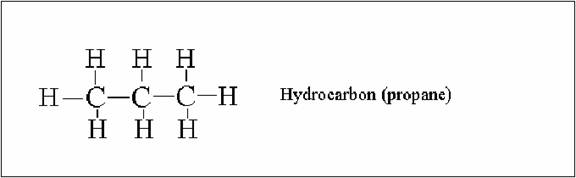
Figure
1: Talbot, Chris. “The Science of Ski Waxes.” (
Before
the use of hydrocarbons, ski racers made their own wax, with conglomerations of
bear fat, honey, sap, and oil, to name a few ingredients. The recipes of waxes were top secret, but
crude (Talbot 3). Today, wax companies
have top-secret recipes of chemicals.
They are sold to whomever wants to buy them (Toko® Tech Manual).
There
are three types of hydrocarbons used in glide wax. Paraffins are chains of 25-35 carbon
atoms. They are soft candle-like waxes
used for warm temperatures. Paraffins
have a low coefficient of friction, meaning that they do not experience much
friction. Because they are such short
chains, snow crystals easily puncture paraffins. This makes them not work well in colder
conditions, when snow crystals are very hard and pointy (Charonnat, Fluorinated
Waxes).
The
diagram below shows a hydrocarbon. The
C's are carbon atoms and the H's are hydrogen atoms. The subscripted numbers next to the carbon
atoms show which carbon atom it is, numbering from left to right. This paraffin has 24 carbon atoms.
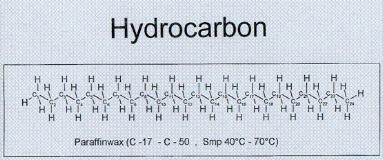
Figure
2: "TokoÒ Chemical Makeup of Glide
Wax" Toko® Information Center
Branched
hydrocarbons with 25-50 carbon atoms are known as microcrystalline. Microcrystalline waxes have a slightly higher
coefficient of friction than paraffins.
This means that they experience more friction than paraffins. Microcrystalline, however, has more strength
than paraffins, so it can be used in colder snow, when snow and ice crystals
are long and sharp. Microcrystalline and
paraffins are often used together in the same wax, because hydrocarbons bond
together fairly well (Charonnat, Fluorinated Waxes).
A
third type of hydrocarbon is synthetic wax.
Synthetic waxes are slightly branched and contain 50-60 carbon
atoms. Because they are so big, they
have a high melting point and are also very hard at skiing temperatures. This makes them work very well on cold snow
(Talbot 10).
Fluorocarbon
waxes are very similar to hydrocarbons, except that fluorine atoms have
replaced some or all of the hydrogen atoms.
Fluorine is the most electronegative element known. This means that fluorine attracts negatively
charged electrons better than element.
This makes fluorine very hydrophobic, which means that it repels
water. Wax companies borrowed the idea
of using fluorocarbons to repel water from fishermen. Fluorocarbons would be painted on the bottoms
of boats to help repel water (Charonnat, Fluorinated Waxes).
The
first fluorocarbon wax was polytetrafluoroethylene, or PTFE. PTFE is also known as Teflonä. PTFE is a branched fluorocarbon chain with
over 500 carbon atoms (Charonnat, Fluorinated Waxes).
The
first commercially available fluorocarbon ski wax was Cera F, produced by
Swix. Cera F is a perfluorocarbon,
meaning that fluorine atoms have replaced all of the hydrogen atoms. Perfluorocarbons usually have around 20
carbon atoms, making their melting points low (Charonnat, Fluorinated Waxes).
At
the 1987 Oberstdorf World Championships, the Italians started using
fluorocarbons. By the 1988 Calgary
Olympics, fluorocarbons were being used extensively (Karlson).
Figure
3, shown below, shows a perfluorocarbon.
The spelling in figure 3 is due to the fact that it was originally made
for Europeans. As with the figure 2,
each C corresponds to a carbon atom, with the subscripts next to the carbons
showing which carbon is there, numbering from left to right. Each F represents a fluorine atom. Because this is a perfluorocarbon, a fluorine
atom has replaced every hydrogen atom.
This fluorocarbon has 14 carbon atoms.
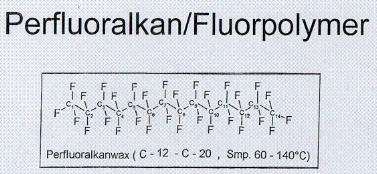
Figure
3: “Toko® Chemical Makeup of Glide
Wax.” Toko® Information Center
Fluorocarbons
have a very negative surface, because of fluorine's high
electronegativity. Fluorocarbon waxes
not only repel water, but also dirt particles, which tend to be negatively
charged. This keeps dirt off of ski
bases. Dirt slows down skis, so this is
important to skiers. This makes
fluorocarbons work very well during spring skiing, when snow tends to be very
dirty (Charonnat, Fluorinated Waxes).
Fluorocarbons
are very expensive, mainly because they are produced at very few places in the
world. Wax companies buy fluorocarbons from
these facilities and then customize them.
Fluorocarbons also lack structural strength, like paraffins (Charonnat,
Fluorinated Waxes). Most fluorocarbons
also have a high melting point. This
means waxing irons must be very hot in order to melt fluorocarbons into skis
(Glenn Randall).
Torbjorn
Karlson, a former US Ski Team coach and a co-founder of fasterskier.com, wrote
of waxing with fluorocarbons in an e-mail interview, "You use high
heat, have to be careful and constantly move the iron - you have to
develop a feel for how to do
this right."
Chlorofluorocarbons, or CFCs, are banned
refrigerants. CFCs contain chlorine,
which makes them damaging to the ozone layer.
Fluorocarbons and chlorofluorocarbons are not the same. Fluorocarbons do not contain chlorine, which
is what makes CFCs damaging to the atmosphere (Charonnat, Understanding Glide
Waxes).
Fluorocarbons and hydrocarbons do not typically
mix well together. This can be compared
to oil and water. The reason is that
hydrocarbons have electrically neutral surfaces, while fluorocarbons have
negatively charged surfaces. Ski bases,
as I have written before, are made with hydrocarbons. In order to mix fluorocarbons and hydrocarbons,
a hybrid is made (Talbot 11).
Fluorinated waxes have one side that is
fluorocarbon, and the other side that is hydrocarbon (Charonnat, Fluorinated
Waxes). Fluorinated waxes are made with
different amounts of fluorine. More
fluorocarbon is not always better. Waxes
for dry, cold conditions fluorinated waxes usually contain much less fluorine
than waxes for warm, wet conditions.
This is because not as much snow melts at the colder conditions, and
substances with more strength than fluorocarbons are needed (Toko® Tech Manual
4).
Figure 4, on the next page, shows how fluorinated
waxes actually consist of a fluorocarbon molecule that is combined with a
paraffin molecule. This figure is also
made for Europeans, so spellings for many words are different, but this figure
illustrates what fluorinated waxes are quite well.
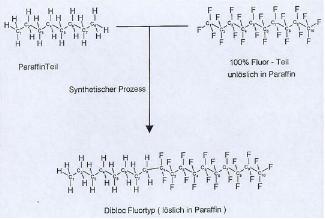
Figure
4: “Toko® Chemical Makeup of Glide
Wax.” Toko® Information Center
Other
types of waxes include graphite waxes and molybdenum waxes. These waxes are mainly used to decrease
static charges and lubricate the ski bottom (Toko® Tech Manual 4). An example of the atomic structures of
graphite and molybdenum waxes are shown in figure 5, below. Graphite is shown on the top, molybdenum
shown on the bottom.
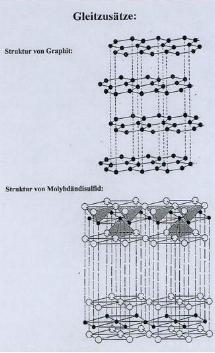
Figure
5: “Toko® Chemical Makeup of Glide
Wax.” Toko® Information Center
Every
skier wants fast skis, whether they are backcountry skiers or Olympic
champions. For backcountry and
recreational skiers, fast skis just make skiing easier, but most of these
skiers do not do too much to make their skis fast. I often see skiers drive down the road with
skis mounted on the top of their vehicle.
This fills the skis with dirt and makes them slower. When these skiers get to the ski trails, many
of them put on their skis and ski through the parking lot. This scratches the bottom of the ski and
fills the base with dirt, both making the ski slower.
Most
of these recreational skiers do not know better than skiing through a parking
lot. Many of them also don't find fast
skis important enough to spend the money to buy wax and then spend the time to
put it on. Some do, but many of these
skiers take their skis to ski shops for a "tuning."
Ski
racers, on the other hand, are willing to do what it takes to have fast
skis. Greg Randall, my coach, told me in
an interview, "There are some races that without fluorocarbons, you would
lose a minute in a 5 km." In a 5 km
race, seconds and tenths of seconds usually are the difference between
skiers. For racers, the threat of losing
a minute or the draw of gaining a minute is well worth expensive fluorocarbon
waxes, or even sending skis to be stone ground by experts with expensive
machines around the country.
Torbjorn
Karlson, in the e-mail interview, stated, "The
best feel is when you out glide your
competition." This draw of having
faster skis than the other racers encourages most skiers to spend many hours
waxing with hydrocarbon training waxes, and then spend more time waxing on
fluorocarbons.
I
have found that having fast skis is very important. Races can be won or lost by the speed of the
skis. While it is the skier on the ski that
is the most important, to win a big race, a skier needs fast skis. While watching the 2003 World Championship
Nordic Combined from Val de Fiem on television, I could tell that Johnny
Spilane, the winner of the race, had much faster skis than anybody around him.
I
have waxed skis a lot. I never knew
quite what I was doing, just that I was making the skis fast. Now I actually know what is happening while I
wax, and what will happen when the ski is on the snow. Skiing fast, for me, has been well worth
every moment spent waxing skis.
My
parents have never let me wax with fluorocarbon waxes. This is because they give off hazardous fumes
when ironed into the ski. Fluorocarbon
waxes can reduce lung function 10-25%.
According to Greg Randall, the effects of waxing with fluorocarbons
without a respirator are, “Sore throat, hacking up white stuff the next day,
and an angry wife.” I have asthma, so
not only would I get the regular ill effects of fluorocarbon fumes, but also
probably have problems with my asthma.
Respirators keep others from having ill effects, but that is a risk that
my parents are not willing to take, especially since we only wax with
fluorocarbons for races, so any ill effects would be during a race the day
after waxing with fluorocarbons.
Greg
Randall uses a respirator when he waxes with fluorocarbons. With a respirator, he feels no ill
effects. Torbjorn Karlson, on the other
hand, says, I used to use one when I daily waxed skis for other skiers. I often
find myself not using one these days since I mostly only wax my own skis and
are not feeling any ill effects. I might go back to using one since it's not
uncomfortable wearing one for that short of a period. It's better to be safe
than sorry later.”
I
have also heard others talk about waxing.
The US Ski Team had people hospitalized last year after they “overdosed”
on fluorocarbons. Now, the US Ski Team
always uses respirators that look more like gas masks. They supposedly have a battery, and I know
that these respirators cover the entire face.
This is much more than the simple respirators my family uses.
Greg
says of waxing with fluorocarbons, “It’s just smoky, and I have stuff all over
my glasses, especially if the iron’s too hot.”
In
my experience with other hydrocarbons, I never have smoke and debris floating
around. I only see wax melt into the
ski, and then solidify.
I
have raced on very fast skis before.
Fast skis make skiing seem effortless.
With fast skis, I have total control of the ski and move very quickly on
uphills, flats, and downhills. Fast skis
also have a special mental advantage.
When I do have fast skis, I know that my skis are going to be as fast if
not faster than anybody’s on the course.
This gives me a confidence boost and makes me more excited for the race.
Slow
skis, on the other hand, are terrible. I
have skied on very slow skis before. I
usually don’t have slow skis other than in training, and then only if I am
about to have a race with entirely different conditions than I am skiing
on. Slow skis break morale and require a
lot of effort.
Everyone
agrees, fluorocarbons are fast. If they
weren’t fast, people wouldn’t be willing to buy them and wax with them. Torbjorn says he waxes with fluorocarbons at
“80-90% of all races.” Greg waxes with
fluorocarbons, “weekly during ski season.”
While
fluorocarbons may be expensive and unpleasant to wax with, they are fast and
give a good control of skis. Most skiers
feel that these benefits outweigh the drawbacks of waxing with fluorocarbons. Waxing with fluorocarbons is just one of the
sacrifices skiers make, just like hard training or long trips to races or snow.
I
predict that in the near future, skis may be become electrical. The electricity would melt a thin layer of
water from the ice on the bottom of the ski.
This would increase the need for fluorocarbons, because there would
never be conditions cold enough to have snow not melt. Synthetic waxes would suddenly become almost
obsolete, because they are for cold conditions, when the snow underneath the ski
does not melt.
Through
the course of writing this paper, I have learned that fluorocarbons work for
real reasons. They do not work with some
sort of magic. Most skiers don’t know
much about how fluorocarbons work, only that they do. I now understand how fluorocarbons work on an
atomic level, microscopic level, and macroscopic level.
I
feel that knowing more about how skis glide and waxes work gives a better
understanding of the entire sport.
Skiing is more than just a recreational activity. It is also a way of life, meeting people, and
thinking. Understanding how a ski
actually glides along the snow gives a greater appreciation of skiing, skiers,
and nature.
Works Cited
Charonnat,
Noel. “Fluorinated Waxes.” Sierra Nordic (
Charonnat,
Noel. “Understanding Glide Waxes.” Sierra Nordic (
“Graphite.” Advanced Dictionary. 1988 ed.
Karlsen,
Torbjorn. E-mail interview.
McKibben,
Bill. “Frozen World.” Cross-Country Skier February, 2004 23.4 http://www.crosscountryskier.com/frozen_world_feb_2004.html.
Onion,
Amanda. “Skiing Science: How the Right Wax Can Make All the
Difference.” ABC News (
Randall, Glenn. Personal experience.
Many cities in many states at many times.
Randall,
Greg. Personal Interview.
Talbot,
Chris. “The Science of Ski Waxes.” (
“Toko®
Chemical Makeup of Glide Wax.” Toko®
Information Center
“Toko® Tech Manual Nordic 2003/2004.” Toko® Information Center 17 pp,